Abstract
Background: T-cell acute lymphoblastic leukemia (T-ALL) accounts for approximately 10-15% or 25% of cases of pediatric or adolescent and young adult (AYA) ALL, respectively. The use of pediatric protocols can improve outcomes in AYA T-ALL. Furthermore, nelarabine (NEL) has been shown to be effective for patients with relapsed or refractory T-ALL. This nationwide, multicenter, prospective, phase II trial for T-ALL was conducted to assess the feasibility and efficacy of NEL, intensive L-asparaginase (L-asp), and protracted intrathecal therapy (IT) when incorporated in the AIEOP-BFM-ALL 2000 based pediatric treatment for patients <25 years old at diagnosis.
Patients & Methods: From December 2011 to November 2017, 364 patients with newly diagnosed T-ALL, age 0-24 (median 9.6 years), were enrolled in the JPLSG ALL-T11/JALSG T-ALL-211-U (ALL-T11) trial conducted by the Japanese Pediatric Leukemia/Lymphoma Study Group and the Japan Adult Leukemia Study Group. Patients were stratified into three groups according to their prednisone (PSL) response, initial central nervous system (CNS) status, and PCR-based minimal residual disease (MRD) at the end of induction consolidation protocol IB (TP2). Good or poor PSL responses were defined as <1.0 × 10 9/L or ≥1.0 × 10 9/L blasts in peripheral blood at day 8 following a 7-day PSL pre-phase plus one IT dose of methotrexate, respectively. Patients with good PSL response, TP2 MRD < 10 −3 and no CNS involvement, were assigned to the standard risk (SR) group, while patients with TP2 MRD ≥ 10 −3 or no complete remission (CR) after induction therapy IA were assigned to the very high risk (VHR) group. The patients who did not fulfill SR and VHR criteria were assigned to the high risk (HR) group. If TP2 MRD evaluation was not applicable, patients were classified as MRD < 10 −3. ALL-T11 was characterized by dexamethasone in IA, the additional use of E. coli derived L-asp (5000 U/m 2 × 8 in IB, 10,000 U/m 2 × 4 in reinduction protocol IIB for SR and HR groups, and 12,500 U/m 2 × 4 in protocol M for SR), incorporation of NEL (5-day course of 650 mg/m 2/day) in HR or VHR groups (6 or 1-2 courses, respectively), and elimination of prophylactic cranial radiotherapy (CRT). CRT was limited to patients with initial CNS involvement (CNS3), and the other patients received protracted IT during each treatment phase including the maintenance phase. Only VHR patients were scheduled to receive hematopoietic stem cell transplantation (HSCT) after being randomized to receive one of two arms of distinct block therapies.
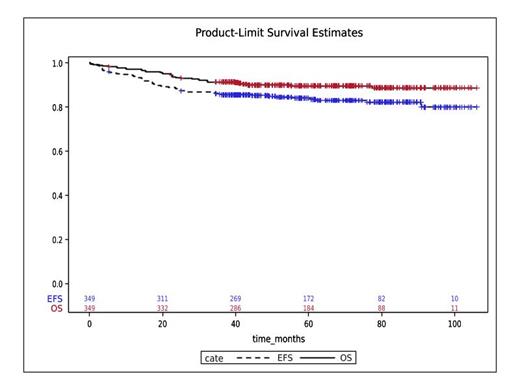
Results: Fifteen patients were excluded having not meeting inclusion criteria. Of 349 evaluable patients, 238 (68.2%) were male, the median white blood cell count was 45 × 10 9/L (range 0.4-1375), and 73.4% were HR by NCI criteria. Twenty-eight patients (8.0%) had CNS3 status. PCR-MRD could be evaluated in 208 patients. Among 310 stratified patients, 168 (54.2%), 103 (33.2%), and 39 (12.6%) were SR, HR, and VHR, respectively. HSCT was performed in 35 patients (10.0%). The composite CR (CR+CR in suppression) rate after IA, and the CR rate after IB were 85.4% and 90.5%, respectively. With a median follow-up of 5 years 2 months, the 3-year event-free survival (EFS) and overall survival (OS) of the whole cohort was 85.6% (95% CI: 81.5-89.9) and 91.4% (87.9-93.9), respectively (Figure 1), and the 3-year cumulative incidence of relapse was 8.9% (5.9-12.1). Induction death was seen in 14 patients (4.0%), and 3-year non-relapse mortality of the whole cohort was 0.6% (0.1-2.1). Three-year EFS and OS for each risk group were 90.4% (84.9-94.0) and 95.8% (91.4-98.0) in SR, 91.3% (83.9-95.4) and 95.1% (88.6-97.9) in HR, and 87.2% (71.9-94.5) and 87.2% (71.9-94.5) in VHR respectively. Three-year EFS and OS were 90.1% (86.1-93.0) and 95.7% (92.7-97.5), and 55.6% (30.5-74.8) and 66.7% (40.4-83.4) in MRD-negative and MRD-positive patients (p < 0.001 and p < 0.001), respectively. Grade 3 or higher peripheral motor and sensory neuropathies were seen in 9 (8.7%) and 6 (5.8%) in HR and 2 (5.1%) and 0 in VHR, respectively. Clinical allergic reaction, anaphylaxis, and pancreatitis were reported in 10 (2.9%), 16 (4.6%), and 31 (8.9%) patients, respectively.
Conclusions: The addition of NEL, intensified L-asp, and protracted IT in AIEOP-BFM-ALL 2000 based treatment showed encouraging outcomes with acceptable toxicities despite the limited use of CRT and HSCT.
Hatta: Bristol-Myers Squibb: Honoraria; Novartis KK: Honoraria; Pfizer Japan Inc.: Honoraria; Otsuka Pharmaceutical.: Honoraria. Imai: Juno Therapeutics: Patents & Royalties: chimeric receptor with 4-1BB signaling domain. Saito: Toshiba corporation: Research Funding. Kiyoi: Astellas: Honoraria; celgene: Honoraria; Daiichi Sankyo: Honoraria; Dainippon Sumitomo: Honoraria; Eisai: Honoraria; Fijifilm: Honoraria; Kyowa Kirin: Honoraria; Otsuka: Honoraria; Perseus Proteomics: Honoraria; Pfizer: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; Takeda: Honoraria; Zenyaku Kogyo: Honoraria. Matsumura: Nippon Shinyaku: Research Funding; Ono: Research Funding; Bristol-Myers Squibb: Speakers Bureau; Daiichi Sankyo: Research Funding, Speakers Bureau; Pfizer: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau; Chugai: Research Funding; Asahi Kasei: Research Funding; Japan Blood Products Organization: Research Funding; Mundipharma: Research Funding; Amgen: Speakers Bureau; AYUMI Pharmaceutical: Research Funding; Eli Lilly Japan: Research Funding; Sumitomo Dainippon: Research Funding; Takeda: Research Funding; Astellas: Speakers Bureau; Kyowa Kirin: Research Funding; Taiho: Research Funding; Nihon Pharmaceutical: Research Funding; Janssen: Speakers Bureau; Mitsubishi Tanabe: Research Funding; Eisai: Research Funding; Otsuka: Consultancy, Research Funding, Speakers Bureau; MSD: Research Funding; Shionogi: Research Funding; Addvie: Research Funding. Miyazaki: Chugai: Honoraria; Kyowa-Kirin: Honoraria; Astellas: Honoraria; Novartis: Honoraria; Abbvie: Honoraria; Sumitomo-Dainippon: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria; Eisai: Honoraria; Janssen: Honoraria; Pfizer: Honoraria; Bristol-Myers Squibb: Honoraria; Nippon-Shinyaku: Honoraria; Takeda: Honoraria; Sanofi: Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal